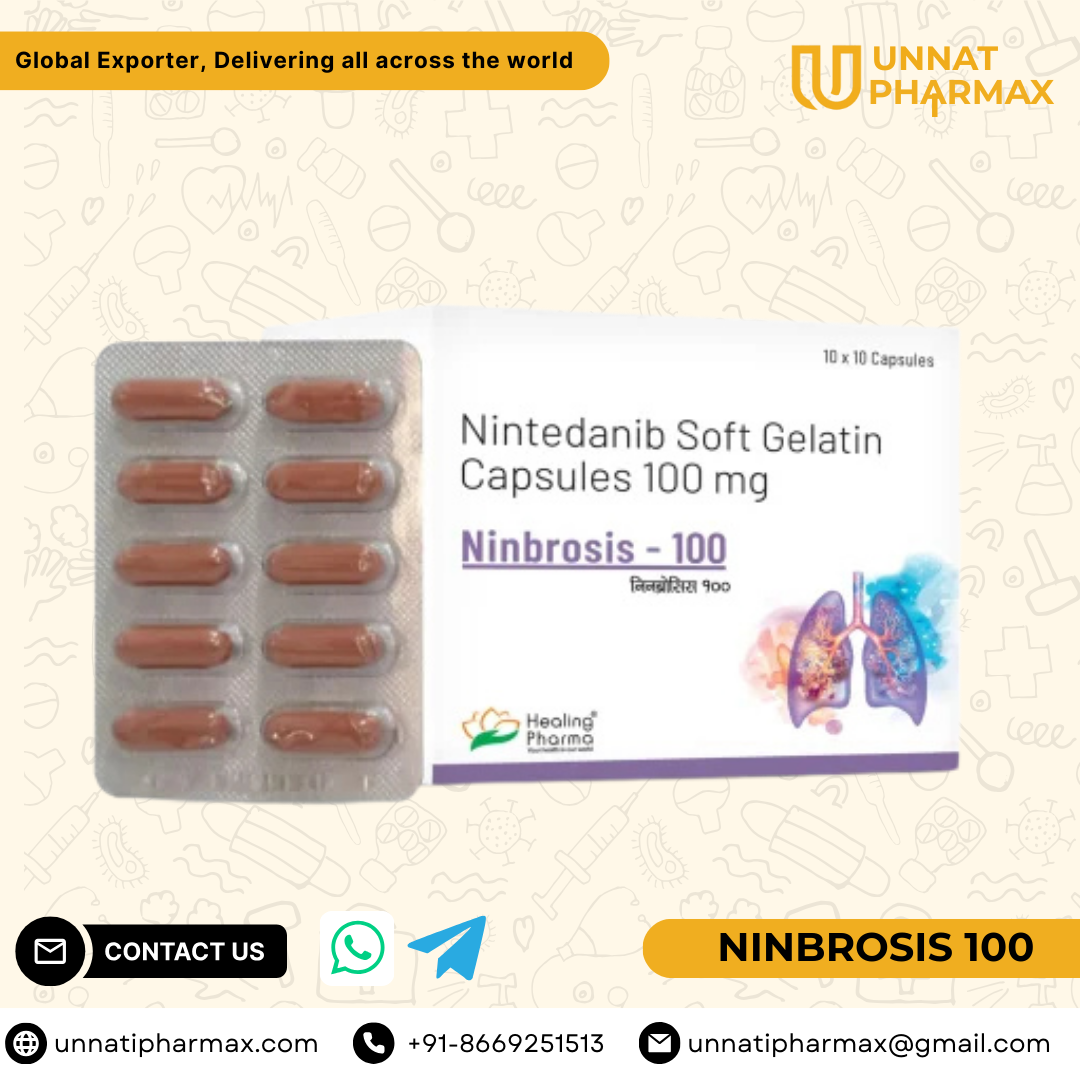
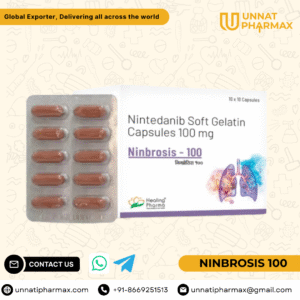
Ninbrosis 100 Capsule
Description:
Ninbrosis 100 Capsule is an anti-fibrotic and anti-cancer medicine that belongs to the group of tyrosine kinase inhibitors. It is primarily prescribed for the treatment of idiopathic pulmonary fibrosis (IPF), a condition that causes scarring of lung tissue leading to breathing difficulties. It is also used in the treatment of certain cancers, including non-small cell lung cancer (NSCLC). Ninbrosis works by blocking specific receptors such as VEGF, FGF, and PDGF, which are involved in fibrosis and tumor growth. This helps in slowing down the progression of the disease and improving the patient’s quality of life.
Key Benefits:
-
Used in the treatment of idiopathic pulmonary fibrosis (IPF).
-
Helps in managing non-small cell lung cancer (NSCLC).
-
Slows down abnormal tissue growth and scarring.
-
Improves lung function and breathing capacity in IPF patients.
Common Side Effects:
-
Diarrhea
-
Nausea or vomiting
-
Abdominal pain
-
Reduced appetite
-
Elevated liver enzymes
-
Fatigue or weakness
Precautions:
-
Not recommended during pregnancy or breastfeeding.
-
Regular monitoring of liver function is necessary.
-
Inform your doctor if you have any heart, kidney, or liver problems.
-
Avoid alcohol and smoking while on this medicine.
FAQs:
Q1: What is Ninbrosis 100 used for?
It is mainly used to treat idiopathic pulmonary fibrosis (IPF) and some cases of non-small cell lung cancer.
Q2: How should I take Ninbrosis 100?
It should be taken exactly as prescribed by your doctor, usually with food and at the same time every day.
Q3: Does Ninbrosis 100 cure IPF or cancer?
No, it does not cure these conditions but helps in slowing disease progression and controlling symptoms.
Q4: What precautions should I take?
Avoid alcohol, maintain a healthy diet, and go for regular liver function tests as recommended.
Q5: Can elderly patients take Ninbrosis 100?
Yes, but dose adjustments may be required depending on their health and liver function.
TRUSTED Anti Cancer MEDICINE PROVIDER – UNNATI PHARMAX
Reference:-
Regulatory Authorities & Official Sources
-
European Medicines Agency (EMA) – Ofev (Nintedanib) EPAR
Provides comprehensive details about Ofev (nintedanib) capsules, including dosing, mechanism of action, and approval for conditions like idiopathic pulmonary fibrosis and fibrosing interstitial lung diseases.
https://www.ema.europa.eu/en/medicines/human/EPAR/ofev European Medicines Agency (EMA) -
EMA – Nintedanib Accord (Generic) EPAR
Regulatory assessment for the generic nintedanib product, confirming bioequivalence to Ofev and approved indications.
https://www.ema.europa.eu/en/medicines/human/EPAR/nintedanib-accord European Medicines Agency (EMA)
U.S. FDA & DailyMed Official Information
-
FDA Label (Ofev Capsules – 100 mg & 150 mg)
Official prescribing information showing recommended dosage (150 mg twice daily with food), dose adjustments, and safety precautions.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/205832s013lbl.pdf FDA Access Data
Clinical and Reference Overviews
-
StatPearls – Nintedanib Clinical Use & Dosing
Clinical summary confirming available capsule strengths (100 mg and 150 mg), standard administration (with food), and guidelines for dose modification due to adverse effects.
https://www.ncbi.nlm.nih.gov/books/NBK585049/




Reviews
There are no reviews yet.